Structure of an Atom
Fundamental Constituents of an Atom
An atom contains three basic particles namely protons, neutrons and electrons. The nucleus of the atom contains protons and neutrons where protons are positively charged and neutrons are neutral. The electrons are located at the outermost regions called the electron shell.
Electron
J. J. Thomson, in 1897, discovered negatively charged particles emitted by the cathode towards the anode in a cathode ray experiment. These negatively charged particles are Electrons.
Protons
Ernest Goldstein, in 1886, discovered that with a different condition in the same chamber, anode emitted positively charged particles known as Canal rays or later named as Protons.
Neutrons
J. Chadwick discovered a subatomic particle with no charge and a mass equivalent to protons in the nucleus of all atoms. These neutrally charged particles are Neutrons.
Atomic Structure of Carbon
The carbon atom contains six protons, six electrons and six neutrons making its mass number at 12.
Atomic Structure of Oxygen
The oxygen atom contains eight protons, eight electrons and eight neutrons making its mass number at 16.
Atomic Structure of Hydrogen
The hydrogen atom (H) contains only one proton, one electron, and no neutrons.
Atomic Structure of Helium
Helium atom contains two protons, two electrons and two neutrons making its mass number at 2.
The Fundamental Unit Of Life Class 9 Notes
Different Models on Structure of an Atom
Since the time of the discovery of atoms, there are a variety of theories which were formulated by many renowned scientists. Mentioned below are the important theories about the structure of an atom as per the chapter.
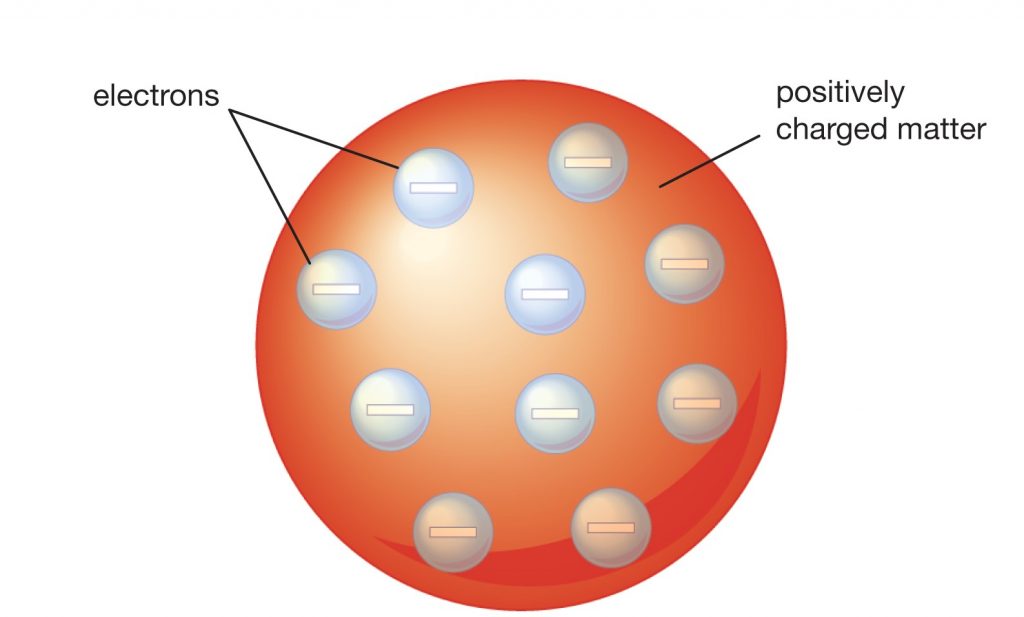
Thomson’s Model of an Atom
J. J. Thomson proposed that the structure of an atom is similar to that of a Christmas pudding where electrons are embedded like currants in the sphere. He proposed that:
- The structure of an atom is a positively charged sphere that embeds electrons in it
- An atom is electrically neutral as the protons and electrons are equal in magnitude

Drawbacks of Thomson’s Model: Thomson’s structure of an atom failed to explain the arrangement of protons and electrons in its structure.
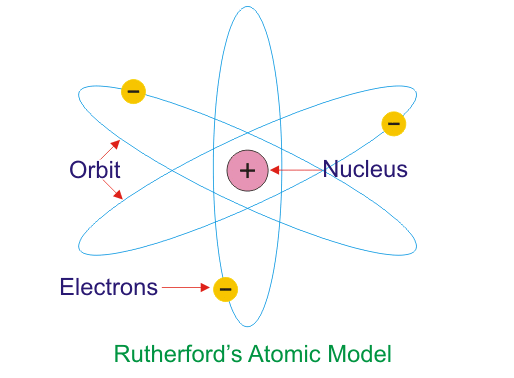
Rutherford’s Model of an Atom
Rutherford conducted an experiment bombarding the alpha (α)-particles on a gold foil. He observed the trajectory of the alpha (α)-particles after passing through an atom and drafted some postulates of the experiment, which are:
- Most of the space in an atom is empty as the particles passed through the gold foil without any hindrance
- The positively charged center is called the Nucleus, and all the mass of an atom resides in the center. The particles deflected 1800 after bombarding the nucleus
- The electrons orbit the center in a defined path
- The size of the nucleus is small compared to the total size of the atom
These were the postulates given by Rutherford using scattering of alpha (α)-particles on a gold foil experiment.
Drawbacks of the Model: Although Rutherford presented an entirely new model regarding the structure of the atom, there were a lot of drawbacks which he failed to explain, are-
- The electrons revolve in an unstable path, and they undergo acceleration radiating energy. When the electrons revolve, they lose energy. Soon electrons would collapse into the nucleus. This tendency would make an atom highly unstable while the atom is highly stable
- Rutherford’s structure of an atom failed to explain the atomic number concept as it explained only the presence of protons in the nucleus
Bohr’s Model of an Atom
Bohr devised a model in order to overcome the objections that Rutherford’s model raised. So, he stated the following postulates:
- An atom permits only a discrete amount of orbitals for the electrons to orbit and make the outer structure of an atom
- While revolving, the negatively charged particles do not lose energy in these orbitals or energy levels
- When the electron jumps from one energy shell to another, a change in magnitude takes place

Bohr’s model gives an elaborative explanation on the structure of an atom and overcomes the objections faced by all the other models on the structure of an atom.
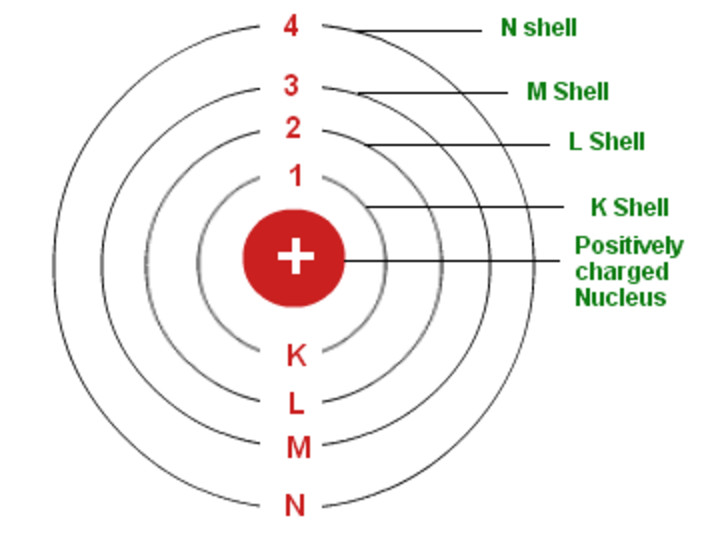
Distribution of Electrons in Distinct Shells
Bohr-Bury Scheme suggested the arrangement of particles in different orbits. The following are the rules to write the number of particles in different orbitals:
- The formula 2n^2 gives the accommodation of the maximum number of electrons in each shell, n=1, 2, 3, 4 for K=2, L=8, M=18, N=32.
- The outermost orbit can hold a maximum of 8 electrons.
- The electrons fill the inner levels first as they follow the stepwise filling of orbitals
Number of electrons in K-shell: n = 1
2n2 = 2 × 12 = 2
Maximum number of electrons in K-shell, first shell = 2
Number of electrons in L-shell, n = 2,
2n2 = 2 × 22 = 8
Maximum number of electrons in L-shell, Second shell = 8
Using the formula 2n^2 number of electrons in any shell can be calculated.
Valency
The next important concept in our notes of the structure of an atom is that of valency. The negatively charged particles present in the outermost shell are called Valence Electrons. These valence electrons are responsible for the valency of an atom.
Valency is the tendency of an atom to react with the other atoms of the same or various elements. The atoms that fill the outermost paths show chemical activity towards other valence electrons. This reactivity is responsible for the formation of molecules between two or more atoms.
The valency becomes zero for an atom when the outer bounds have eight electrons or no electrons to lose. The particle with eight electrons in the outermost shell is an octet, and these molecules are mostly inert in nature.
Magnesium (Mg) has a configuration (2, 8, and 2), so the valency is two. Oxygen (O) (2, 8, and 6) has the valency two as the number electrons it can gain is two to achieve a packed outer energy level. Similarly, Helium (He) has 2 electrons in its outer shell, Neon (Ne) (2, 8, and 8) has eight electrons in its outer shell. Hence, they do not show any chemical activity.
Examples of Chemistry in Everyday Life
Atomic Number (Z)
The nucleus of an atom consists of Protons, and the atomic number is equal to the number of protons present in one atom of an element. As the atom is electrically neutral, the number of protons and electrons are the same. The notation Z denotes an Atomic number. The atomic number of Hydrogen is one as it has only one proton.
Grasping these essential points of the chapter structure of an atom will be helpful for you-
Number of Protons present in an atom = Atomic number (Z)
Number of Electrons present in an atom= Atomic number (Z)
Number of Neutrons = Mass number (A)- Atomic number (Z)
Mass Number (A)
The mass number is the measure of the total number of protons and neutrons in the nucleus of an atom. The notation A indicates the Mass number. The notation N signifies the total number of neutrons.
Mass Number = Atomic Number + Number of Neutrons in the Nucleus
A = Z + n°
Mass Number is also called Nucleon number.
Isotopes and Isobars
Isotopes and Isobars are important concepts that you must understand for getting a better grip over the chapter.
Isotopes
The atoms of the same elements with the same atomic number and different mass numbers. Hydrogen has three isotopes: Protium, Deuterium, Tritium.
Examples:
Isobars
The atoms of different molecules with the same mass number. For Example, in Calcium, atomic number 20, and argon, atomic number 18, the mass number of both these elements is 40. This shows that the total number of nucleons is the same in the atoms.
Examples:
Important Questions and Answers
Q1. State the properties of electrons, protons, and neutrons.
Sol:
| Property | Electrons | Protons | Neutrons |
| Charge | Negatively Charged | Positively Charged | No Charge |
| Affinity | Attracts to positively charged | Attracts to negatively charged | Get attracted neither to positive nor negative |
| Weight | Mass is negligible | 1 a.m.u | 1 a.m.u |
| Location | Outside the nucleus | Within the nucleus | Inside the nucleus |
Q2. Describe the limitations of J.J Thomson’s model of the atom.
Sol: According to this model, the electrons are embedded all over in the positively charged spheres. But experiments showed that protons are only present in the center of an atom and electrons are distributed around the nucleus of an atom.
Q3. State the limitations of Rutherford’s model of the atom.
Sol: According to this model, the electrons revolve around a circular orbit around the nucleus. Any such particle that revolves around the nucleus would undergo acceleration and radiate energy. The revolving electron would lose its energy and finally fall into the nucleus, the atom would be highly unstable. Though, the atoms are quite stable.
Q4. Describe Bohr’s model of the atom.
Sol: Check the following statements:
- An atom has a nucleus in the centre.
- Negatively charged electrons revolve around the nucleus.
- The atoms of the nucleus contain distinct orbits of electrons.
- Electrons do not radiate energy when revolving in the distinct orbits
- These orbits or shells are represented by the letter K,L,M,N or the numbers 1,2,3,4.
Q5. State comparison of all the proposed models of an atom given in this chapter.
Sol:
| Thomson | Rutherford | Bohr |
| The sphere is positively charged. | The positively charged sphere in the center is called the nucleus. | The positive charge at the center is called the nucleus. |
| Electrons spread randomly all over the sphere. | Electrons revolve around the nucleus in the orbits. | Electrons revolve in discrete orbits and do not radiate energy. |
| Positive Charge = Negative Charge. Atom is electrically neutral. | The size of a nucleus is very small as compared to the size of an atom. | The orbits were termed as energy shells by the letter K, L, M, N, or the numbers 1,2,3,4. |
Credits: Britannica
Credits: COCC
Credits: Class notes
Q6. Summarise the rules for the writing of the distribution of electrons in various shells for the first eighteen elements.
The rules for the writing of the distribution of electrons in various shells for the first eighteen elements are:
(i) The maximum number of electrons present in a shell is a formula—2n2 n = orbit number i.e., 1, 2, 3 Maximum number of electrons in different shells are: K shell n = 1 2n2 ⇒ 2(1)2 = 2 L shell n = 2 2n2 ⇒ 2(2)2 = 8 M shell n = 3 2n2 ⇒ 2(3)2 = 18 N shell n = 4 2n2 ⇒ 2(4)2 = 32
(ii) The maximum number of electrons that can be accommodated in the outermost orbit is 8.
(iii) Electrons are not accommodated in a given shell unless the inner shells are filled. (Shells are filled step-wise).
.png)

