Electromagnetic Spectrum
The sun is our planet’s principal source of energy, and its energy travels in the form of electromagnetic radiation. Electromagnetic energy moves across empty space at the speed of light in the form of waves of electric and magnetic fields with a range of frequencies or wavelengths.
Electromagnetic radiation is a common occurrence in our daily lives. All electromagnetic waves, from visible light, that our eyes can detect to microwave radiation that heats our meals or radio waves that power our radios, X-rays that enable doctors to identify any injury in our bones or UV radiation emitted by a hot surface, are EM waves.
Electromagnetic Waves
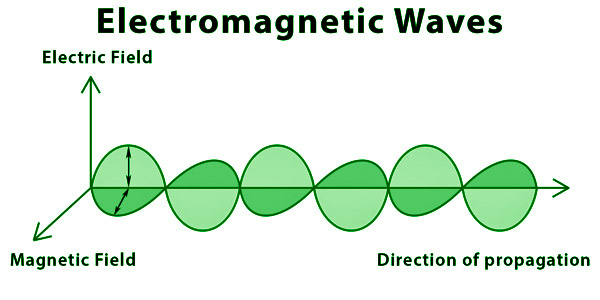
Waves created by the interaction of vibrating electric and magnetic fields are known as electromagnetic waves. An oscillating electric and magnetic field makes up EM waves.
A charged particle, in general, produces an electric field. This electric field exerts a push on other charged particles. Positive charges accelerate in the field’s direction, whereas negative charges accelerate in the opposite direction of the field. A travelling charged particle creates the magnetic field. This magnetic field exerts a push on other moving particles. Because the force acting on these charges is always perpendicular to their movement, it only affects the direction of the velocity, not the speed. As a result, a speeding charged particle generates the electromagnetic field. Electromagnetic waves are nothing more than electric and magnetic fields travelling at the speed of light c through open space.
The sun is our planet’s principal source of energy, and its energy travels in the form of electromagnetic radiation. Electromagnetic energy moves across empty space at the speed of light in the form of waves of electric and magnetic fields with a range of frequencies or wavelengths.
When a charged particle oscillates about an equilibrium location, it is said to be accelerating. If the charged particle’s oscillation frequency is f, it creates an electromagnetic wave of frequency f. The wavelength of this wave may be calculated using the formula:
λ = c/f
Electromagnetic waves are a type of energy transfer that occurs in space.
Representation of Electromagnetic Waves
Electromagnetic Spectrum
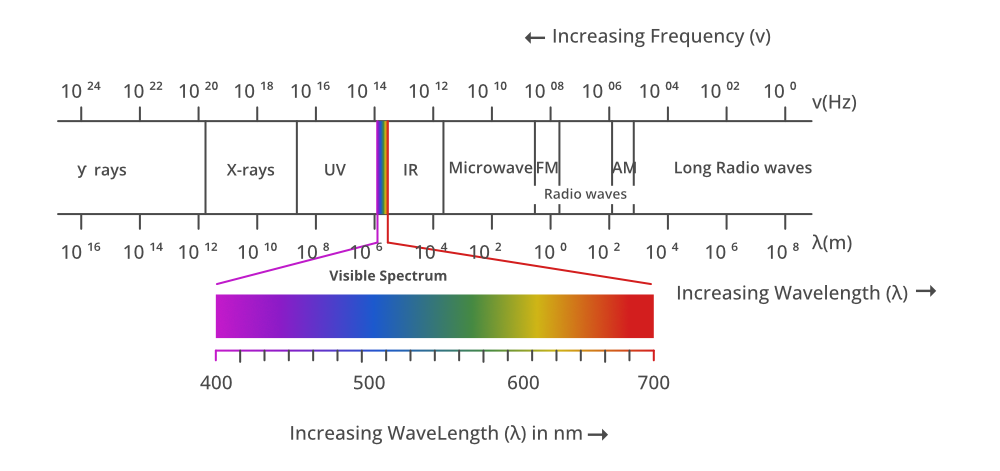
The electromagnetic spectrum is a collection of frequencies, wavelengths, and photon energies of electromagnetic waves spanning from 1Hz to 1025Hz, equivalent to wavelengths ranging from a few hundred kilometres to a size smaller than the size of an atomic nucleus. The electromagnetic spectrum can thus be described as the range of all types of electromagnetic radiation in basic terms. In a vacuum, all electromagnetic waves travel at the same speed as light. For different forms of electromagnetic waves, however, the wavelengths, frequencies, and photon energy will vary.
Terms related to Electromagnetic Waves
The frequency (f), wavelength (λ), energy (E) of an electromagnetic wave are related to each other as:
λ=c/f
f=E/h
E=hc/λ
where
- c=3×108m/s represents the speed of light in a vacuum
- h=6.626×10–34J.s represents Planck’s constant.
Electromagnetic Waves in Electromagnetic Spectrum
Radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays, gamma rays, and cosmic rays make up the full range (electromagnetic spectrum) in decreasing order of frequency and rising order of wavelength.
- Radio Waves
- The rapid travel of charged particles across conducting wires causes these waves.
- Radio, television, and telecom signals are transmitted through them.
- These waves have a frequency range of around 3kHz to 300MHz.
- In the ultrahigh-frequency (UHF) band, cellular phones employ radio waves to convey voice communication.
- Radio picks up radio waves that are broadcast by radio stations. Radio waves can be emitted by gases and stars in space. The majority of radio waves are used for TV and mobile communication.
- Microwaves
- Microwaves are a type of electromagnetic radiation that has a frequency of a few gigahertz (GHz).
- Klystrons, magnetrons, and Gunn diodes are unique vacuum tubes that produce them.
- Microwaves are commonly utilised in aviation navigation due to their short wavelengths.
- These rays are employed in microwaves, which aid in the heating of meals in homes and offices. It’s also used by astronomers to figure out and understand the structure of surrounding galaxies and stars.
- Infrared Rays
- Infrared waves are produced by hot bodies and molecules and are thus referred to as heatwaves.
- Infrared rays are near the low-frequency or long-wavelength end of the visible light spectrum.
- The greenhouse effect caused by these rays is critical for maintaining global warming and average temperatures.
- Greenhouse gases such as carbon dioxide and water vapour trap these radiations in the earth’s atmosphere.
- Night vision goggles make use of these radiations. Infrared light generated by objects in the dark can be read and captured by these devices. Infrared light is used to trace interstellar dust in space. Infrared radiation is emitted by electronic devices and is commonly employed in remote switches for a variety of household gadgets.
- Visible Rays
- Visible rays are electromagnetic waves that can be seen with the naked eye. They are the most common type of electromagnetic waves.
- These can be found in the frequency range of 4×1014Hz–7×1014Hz or the wavelength range of 400nm–700nm.
- The visible light rays reflected or released from the objects around us assist us in seeing the world, and the range of visible radiation is different for different creatures.
- Devices that emit light in the visible area of the electromagnetic spectrum include bulbs, lamps, candles, LEDs, tube lights, and so on.
- Ultraviolet Rays
- Although the sun is the primary source of ultraviolet radiation on Earth, the ozone layer absorbs the majority of UV energy before it reaches the atmosphere.
- UV radiation has a wavelength of 400nm–1nm.
- These radiations are emitted by special lamps and extremely hot bodies, and in big numbers, they can cause significant injury to humans. It tans the skin and creates burns.
- Because these radiations may be focused on tiny beams, they are used in high precision applications such as LASIK or laser-based eye surgery.
- UV lamps are used in water purifiers to eliminate microorganisms that may be present in the water.
- When working with UV welding arcs, welders use special goggles to protect their eyes.
- X-Rays
- This electromagnetic radiation is found outside of the ultraviolet (UV) region of the electromagnetic spectrum and is extremely valuable in the medical field.
- The wavelength range of X-ray radiation is 1nm–10–3nm.
- By blasting a metal target with high-energy electrons, X-rays can be produced.
- X-rays are a diagnostic technique in medicine that can be quite helpful in the treatment of some types of cancer. To find the source of the problem, a doctor utilises an x-ray scanner to scan our bones or teeth. Overexposure to x-rays can cause harm or death to the organism’s healthy tissues. As a result, extreme caution must be exercised when dealing with x-rays.
- At the airport checkpoint, security agents utilise it to search through passengers’ luggage. X-rays are also emitted by the universe’s heated gases.
- Gamma-Rays
- The universe is the largest gamma-ray generator.
- These rays are in the electromagnetic spectrum’s higher frequency region.
- Gamma rays have wavelengths ranging from 10–12m to 10–14m.
- Radioactive nuclei release high-frequency radiations, which are also created during nuclear processes.
- Gamma rays have a wide range of medical applications, including the destruction of cancerous cells. Gamma-ray imaging is a technique used by doctors to examine the insides of patients’ bodies.
Types of Radiation | Frequency range (Hz) | Wavelength Range |
Gamma-rays | 1020-1024 | <10-12 m |
X-rays | 1017-1020 | 1 nm – 1 pm |
Ultraviolet rays | 1015-1017 | 400 nm – 1 nm |
Visible rays | 4 x 1014 – 7.5 x 1014 | 750 nm – 400 nm |
Near-infrared | 1 x 1014 – 4 x 1014 | 2.5 μm – 750 nm |
Infrared rays | 1013 – 1014 | 25 μm – 2.5 μm |
Microwaves | 3 x 1011 – 1013 | 11 mm – 25 μm |
Radio waves | < 3x 1011 | >1 mm |
Spectroscopy
In terms of wavelength or frequency, spectroscopy is a method for determining the emission and absorption of light and other radiation as it interacts with matter.
As a ray of light passes through matter, it is scattered. It interacts with atoms and molecules of the given substance, and these atoms interact with light waves of similar frequencies based on their resonance frequencies. When light rays collide with an atom in an excited state, certain distinctive frequencies are released, resulting in a line spectrum. This line spectrum is made up of a collection of emission lines that isn’t continuous. The wavelengths of the light produced are separated. When light with continuous wavelengths is passed through a low-density material, an absorption spectrum is created. Atoms and molecules with characteristic frequencies similar to light waves will be absorbed, resulting in a continuous spectrum with a few lines missing.
Applications of Electromagnetic Spectrum
The presence of the full electromagnetic spectrum was originally demonstrated by Maxwell. His mathematics suggested that electromagnetic radiation may have an endless number of frequencies. The electromagnetic spectrum is a frequency and wavelength-based organisation of various radiations. The following are some examples of EM-spectrum applications:
- Hertz was the first to discover radio waves and microwaves. Wireless television and radio, as well as mobile communication, arose as a result of these waves.
- Ultraviolet radiation is useful for the ionisation of atoms, which aids in the initiation of numerous chemical reactions.
- The gamma rays were discovered by Paul Villard. These are employed in the development of nuclear medicine and ionisation experiments.
- X-rays were invented by Roentgen. These are used to discover problems with the bones and teeth, as well as abnormalities.
- The visible light portion of the electromagnetic spectrum allows us to see the world around us. This part of the electromagnetic spectrum aids in the perception of all objects, including colours.
.png)

